Introduction:
Chronic Myeloid leukemia (CML) is a chronic myeloproliferative neoplasm with an increased undifferentiated proliferation of granulocytic lineage of the white blood cells. It is an acquired disorder of the hematopoietic stem cells due to a cytogenetic aberration known as the Philadelphia chromosome. It occurs due to a phenomenon of a reciprocal translocation between chromosomes t(9,22) and results in BCR/ABL fusion gene. The product of this gene has tyrosine kinase property, which predominantly causes the disease. Tyrosine kinase inhibitors (TKI) are the mainstay of the treatment to achieve remission, while bone marrow transplant is the only proven cure.
Tuberculosis (TB), both active and reactivation of latent TB, remains the most common infectious disease worldwide and leads to high mortality. Currently, one-fourth of the global population has been infected by Mycobacterium tuberculosis. The incidence of TB has been reportedly increasing in patients with cancer. It has been mostly found in association with non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Recently, the incidence increased in patients with CML during treatment with Imatinib (IM) has also been reported in a retrospective cohort study in Taiwan by Lui C-J et. al. and few other published cases in literature. This might be due to impairment of the T lymphocytes signal transduction leading to increased host susceptibility to TB, hepatitis B, and varicella-zoster infection.
TB treatment is mainly based on the susceptibility of the Mycobacterium Tuberculosis to the anti-TB medications. First-line therapy includes of four drugs, Isoniazid (INH), Rifampicin (RIF), Pyrazinamide (PZA), and Ethambutol (EMB). Anti TB drugs administration also needs close monitoring because of potential side effects. These include, but not limited to hepatoxicity, optic neuritis, impaired color vision, peripheral neuropathy, and gastrointestinal disturbances. Their occurrence necessitate discontinuation of the culprit drug from the regimen. Certain anti TB drugs are also known to disrupt bioavailability of other drugs metabolized in the liver when concomitantly administered by influencing the hepatic cytochrome P450 enzyme. This happens while using anti-TB medication to treat concomitant TB infection in CML patients on tyrosine kinase inhibitors therapy.
Methods and critical analysis of the reviewed study articles:
The authors reviewed the current literature focusing on all articles addressing TB infections and their diagnosis and management in patients with CML (PubMed and Google Scholar between 2000 and 2020). Our review aimed to provide the necessary data to improve clinical diagnosis and clarify the management strategy of this condition.
Conclusion and treatment proposal:
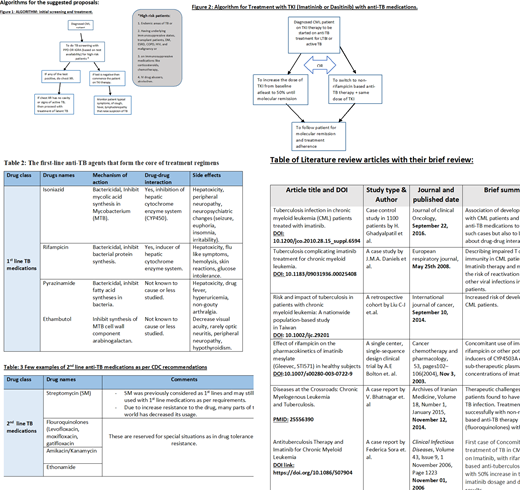
Throughout our review of multiple research articles, it has been realized that patients with CML and other hematological malignancies and solid tumors (e.g., non-Hodgkin lymphoma, chronic lymphocytic leukemia, metastatic gastric tumors), have a higher risk of having TB infection. TB infection (pulmonary and extra-pulmonary) occurs 2-9 times more than the general population in patients with hematological malignancies. In CML patients, treatment therapy with Imatinib posed a higher risk of development of TB due to defective cellular immunity.
Co-administration of 1st line anti Tb medications mainly rifampicin with standard TKIs therapy Imatinib and Dasatinib has resulted in sub-therapeutic levels of these drugs due to induction of CYP4503A enzyme. Either by changing the TB treatment regimen to a non-rifampicin based therapy line with a combination of a fluoroquinolone, increasing the dose, or switching to a different TKI class may result in clinical improvement.
To the best of our knowledge, there are no randomized control trials, or FDA approved guidelines to treat concomitant TB infection in CML patients. Therefore, a clear strategy to deal with such a clinical condition is lacking. The immediate treatment for the concomitant disease has been suggested based on clinical experience and clinical response of some patients published in few case reports, case series, and retrospective cohort studies. After careful literature review, we put forth our management proposals, However it need further research studies and clinical trials to bring recommendations and guidelines.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal